This software is intended for investigators planning a confirmatory trial where it’s suspected that a subpopulation may benefit more than the overall population. The subpopulation could be defined by a risk score or biomarker measured at baseline. The subpopulation must be defined in advance, e.g., based on prior data or medical knowledge. Adaptive enrichment designs have potential to provide stronger evidence than standard designs about treatment benefits for the subpopulation, its complement, and the combined population.
Adaptive enrichment designs have a preplanned rule for changing enrollment criteria based on accrued data in an ongoing trial; for example, future enrollment may be restricted to a subpopulation if the complementary subpopulation is not benefiting. This software tool can help in planning such a trial, by
- tailoring an adaptive enrichment design to the scientific goals and logistical constraints of the investigator, and
- comparing performance of the adaptive design to more traditional designs.
The software searches over hundreds of candidate adaptive designs with the aim of finding one that satisfies the user’s requirements for Type I and II error at the minimum cost. This requires substantial computation and is typically completed within 24 hours, at which time a summary report is emailed to the user.
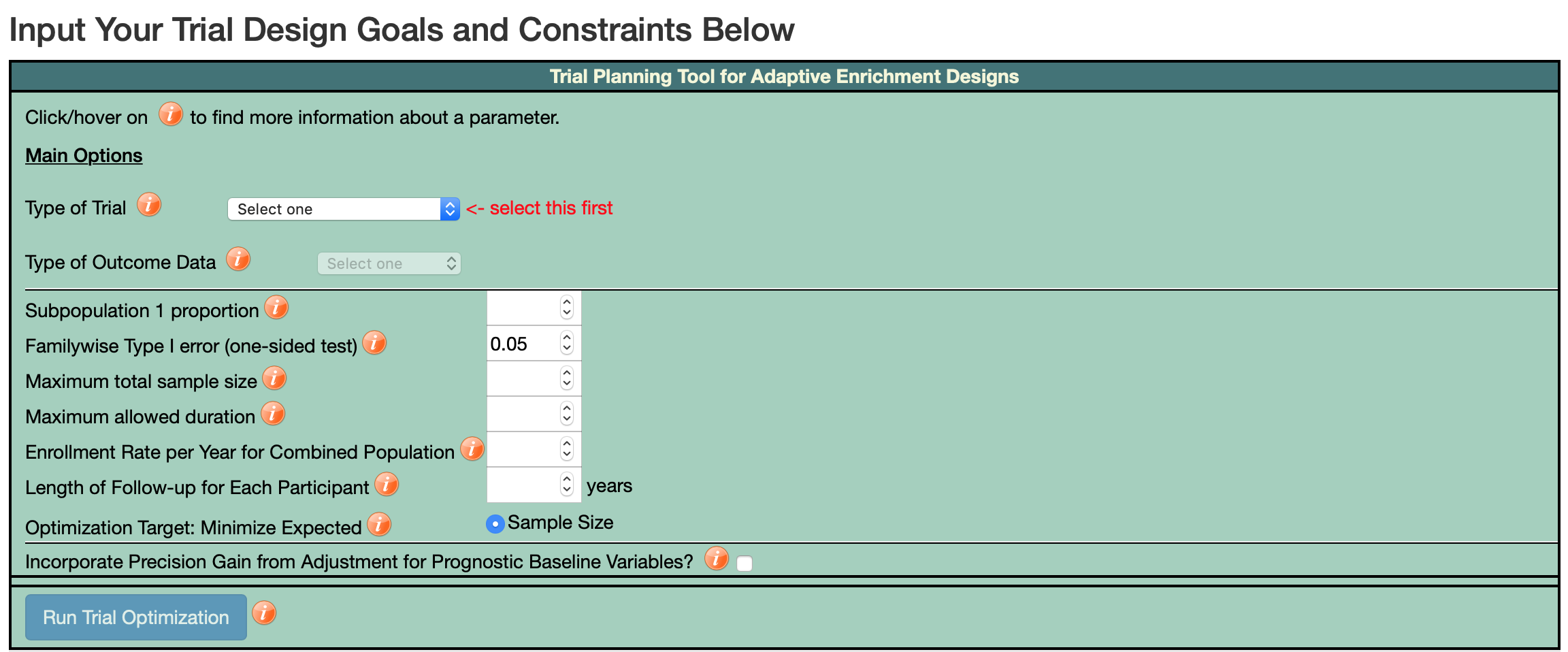
To access the graphical user-interface for this software, please set up a free account here. After creating an account (which may take up to the next business day
to be approved) and logging in, you can use the trial design optimizer by clicking on the drop-down menu "Design Optimizer" at the top of the webpage. An example of the user-interface is below.
Alternatively, the open-source R software and statistical methods papers that it implements are given in the links below
- Code: The source code for this trial design optimizer is available here: Source Code
- An R package version is available here: R Package
- SAS users can run the code via SAS/IML using the code here: SAS code
- R Code to estimate relative efficiency due to adjusting for prognostic baseline variables, using earlier phase randomized trial data set.
- Details of Adaptive Design Enrollment Modification Rules and Multiple Testing Procedures:
- 1. (For 1 treatment versus control): Betz, Josh; Steingrimsson, Jon Arni; Qian, Tianchen; and Rosenblum, Michael, COMPARISON OF ADAPTIVE RANDOMIZED TRIAL DESIGNS FOR TIME-TO-EVENT OUTCOMES THAT EXPAND VERSUS RESTRICT ENROLLMENT CRITERIA, TO TEST NON-INFERIORITY (September 2017). Johns Hopkins University, Dept. of Biostatistics Working Papers. Working Paper 289.
- 2. (For 2 treatments versus control): Steingrimsson, Jon Arni; Betz, Joshua; Qian, Tiachen; and Rosenblum, Michael. OPTIMIZED ADAPTIVE ENRICHMENT DESIGNS FOR MULTI-ARM TRIALS: LEARNING WHICH SUBPOPULATIONS BENEFIT FROM DIFFERENT TREATMENTS (September 2017). Johns Hopkins University, Dept. of Biostatistics Working Papers. Working Paper 288.